
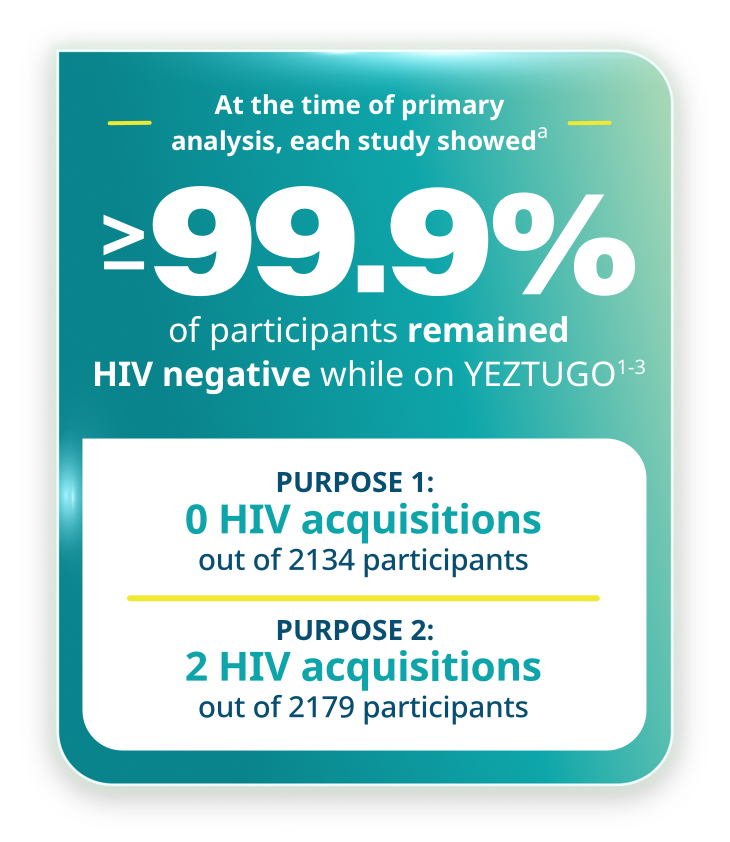
aThe determination of efficacy was based on interim analyses when 50% of randomized participants completed 52 weeks of follow-up. Based on the results, the study arms were unblinded, and this interim analysis became the primary analysis.
Both phase 3 trials evaluated the efficacy of YEZTUGO by comparing the incidence of HIV with the estimated bHIV in the screened population and evaluated the efficacy and safety of YEZTUGO compared with FTC/TDF.2,3
bN values represent the modified intention-to-treat analyses that excluded participants with HIV at baseline.
YEZTUGO was studied across the most age-, gender-, and racially inclusive, phase 3, PrEP clinical trials to date1-5
The first phase 3 HIV prevention clinical trial to study pregnant and lactating women1,2,4,c

cThe first phase 3 HIV prevention clinical trial to allow individuals to stay enrolled and continue on study drug if they became pregnant or started lactating.
dEligible individuals had sexual encounters without recent HIV testing and had unknown HIV status.
eThe determination of efficacy was based on interim analyses when 50% of randomized participants completed 52 weeks of follow-up. Based on results, the study arms were unblinded, and this interim analysis became the primary analysis.
Baseline characteristics2
characteristics
N=2138
N=1070
| Median—yr (range) | 21 (16–25) | 21 (16–25) | |
| 16 or 17 yr—no. (%) | 56 (2.6) | 23 (2.1) |
| Black race— no. (%)f |
2135 (99.9) | 1068 (99.8) |
| Chlamydia trachomatis | 520 (24.3) | 263 (24.6) |
|
| Neisseria gonorrhoeae | 197 (9.2) | 90 (8.4) | |
| Trichomonas vaginalis | 154 (7.2) | 82 (7.7) | |
| Syphilis | 57 (2.7) | 29 (2.7) |
fRace was reported by the participants. All non-Black participants were multiracial.
The first phase 3 HIV prevention study to intentionally include gender-diverse individuals1,3,5

gEligible individuals had condomless sex, no recent HIV testing, and had unknown HIV status.
hThe determination of efficacy was based on interim analyses when 50% of randomized participants completed 52 weeks of follow-up. Based on results, the study arms were unblinded, and this interim analysis became the primary analysis.
Baseline characteristics3
characteristics
N=2138
N=1088
| Median—yr (range) | 28 (17–74) | 29 (17–73) | |
| 16 to ≤25 yr—no. (%) | 752 (34.4) | 344 (31.6) |
| Argentina | 161 (7.4) | 64 (5.9) | |
| Brazil | 769 (35.2) | 396 (36.4) | |
| Mexico | 8 (0.4) | 4 (0.4) | |
| Peru | 309 (14.2) | 138 (12.7) | |
| South Africa | 246 (11.3) | 112 (10.3) | |
| Thailand | 250 (11.5) | 139 (12.8) | |
| United States | 440 (20.2) | 235 (21.6) |
| Asian | 269/2175 (12.4) | 144/1086 (13.3) | |
| Black | 811/2175 (37.3) | 420/1086 (38.7) | |
| Indigenous or Indigenous ancestry | 341/2175 (15.7) | 156/1086 (14.4) | |
| White | 722/2175 (33.2) | 344/1086 (31.7) | |
| Other and other multiracial | 32/2175 (1.5) | 22/1086 (2.0) | |
| Hispanic or Latine | 1378/2182 (63.2) | 675/1088 (62.0) |
| Cisgender man | 1697 (77.7) | 846 (77.8) | |
| Transgender woman | 315 (14.4) | 161 (14.8) | |
| Transgender man | 29 (1.3) | 14 (1.3) | |
| Gender nonbinaryj | 136 (6.2) | 63 (5.8) | |
| Otherk | 6 (0.3) | 4 (0.4) |
| No previous HIV testing—no. (%) | 597 (27.3) | 306 (28.1) | |
| Median time since last HIV test—mo (range)l | 7.2 (2.6–149.4) | 7.1 (1.2–274.2) |
| Any previous use of PrEP—no. (%) | 515 (23.6) | 249 (22.9) | |
| Median time since last use of PrEP—mo (range)m | 13.0 (0.7–103.9) | 10.8 (0.7–274.5) |
| Condomless receptive anal sex with ≥2 partners in previous 12 wks—no. (%) | 2128 (97.5) | 1049 (96.4) |
| Chlamydia trachomatis | 253 (11.6) | 126 (11.6) | |
| Neisseria gonorrhoeae | 193 (8.8) | 115 (10.6) | |
| Syphilis | 84 (3.8) | 43 (4.0) |
| Use of gender-affirming hormone therapy—no. (%)o | 253 (11.6) | 131 (12.0) |
iRace and ethnic group were reported by the participants. The “Black” category included all of the participants who identified as being Black or as being of Black ancestry and included the terms “Black,” “Black/White,” “Black/Pardo” (Brazilian term for a specific racial category), “Black/Brown” (Brazil), “Black/Colored” (South African term for a specific racial category), “Black/American Indian or Alaska Native,” “Black/Asian,” and “Black/Native Hawaiian or Pacific Islander.” The “Indigenous or Indigenous ancestry” category included the terms “American Indian or Alaska Native,” “Native Hawaiian or Pacific Islander,” “Asian/Native Hawaiian or Pacific Islander,” “White/Native Hawaiian or Pacific Islander,” and “White/American Indian or Alaskan Native.” The “other and other multiracial” category included the terms “Asian/White,” “Colored” (South Africa), “Pardo” (Brazil), “White/Brown” (Brazil), “multiracial any other,” and “not multiracial other.”
jAmong the participants who identified as gender nonbinary, 122 (89.7%) in the YEZTUGO group and 53 (84.1%) in the FTC/TDF group were assigned male at birth.
kThe “other” category included participants who identified as “Travesti” (3 participants in the YEZTUGO group and 3 in the FTC/TDF group) or as an “other” gender (3 in the YEZTUGO group and 1 in the FTC/TDF group).
lData are included for 1585 participants in the YEZTUGO group and 782 in the FTC/TDF group.
mIncluded are participants who were not taking PrEP at baseline (449 in the YEZTUGO group and 215 in the FTC/TDF group).
nChlamydia trachomatis and Neisseria gonorrhoeae diagnoses were based on testing of pharyngeal, rectal, and urethral (urine) samples, performed by central and local laboratories. Blood testing for syphilis was performed locally with the use of local testing protocols.
oUse of gender-affirming hormone therapy included concomitant use with the trial regimen during the randomized, blinded phase.

Proven HIV prevention across the broadest range of individuals1-5
YEZTUGO showed superior efficacy compared to FTC/TDF1,2
PURPOSE 1 included cisgender adolescent girls and young women.1,2
Efficacy endpoint: HIV incidence rate/100 PY
- Primary efficacy analysis: HIV incidence rate with YEZTUGO (0/100 PY) was significantly lower than bHIV (2.41/100 PY), IRR (95% CI): 0.000 (0.000, 0.042), p<0.00012
- Secondary efficacy analysis: HIV incidence rate with YEZTUGO (0/100 PY) was significantly lower than FTC/TDF (1.69/100 PY), IRR (95% CI): 0.000 (0.000, 0.101), p<0.00011,2
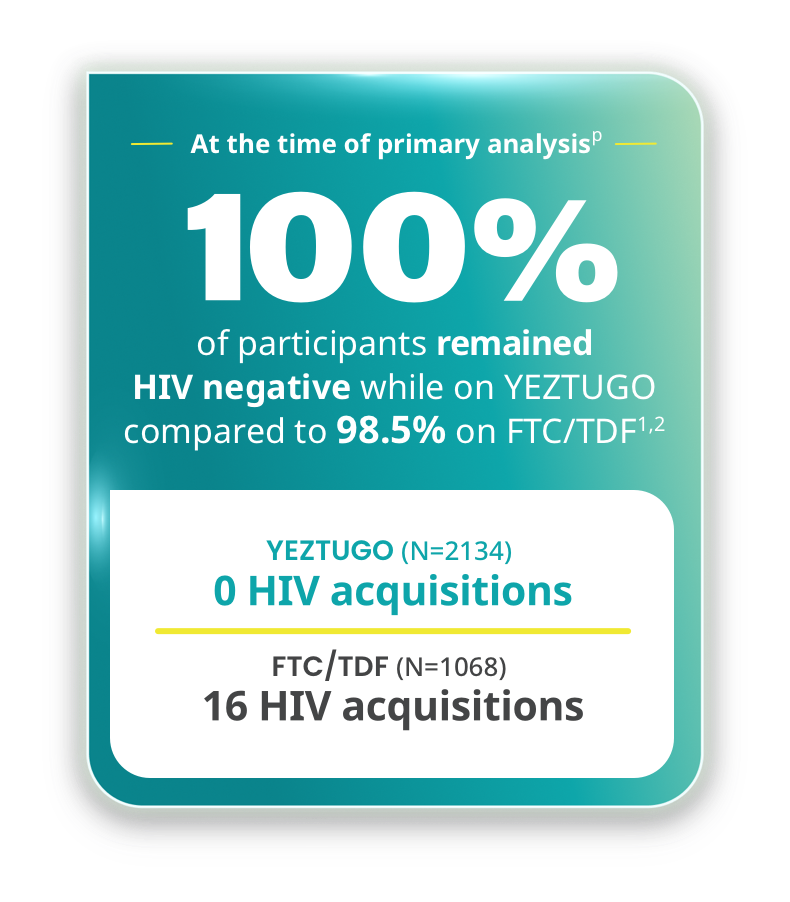
pThe determination of efficacy was based on interim analyses when 50% of randomized participants completed 52 weeks of follow-up. Based on results, the study arms were unblinded, and this interim analysis became the primary analysis.
YEZTUGO showed superior efficacy compared to FTC/TDF1,3
PURPOSE 2 included cisgender men, transgender men and women, and nonbinary persons.1,3
Efficacy endpoint: HIV incidence rate/100 PY
- Primary efficacy analysis: HIV incidence rate with YEZTUGO (0.1/100 PY) was significantly lower than bHIV (2.37/100 PY), IRR (95% CI): 0.043 (0.010, 0.182), p<0.00013
- Secondary efficacy analysis: HIV incidence rate with YEZTUGO (0.1/100 PY) was significantly lower than FTC/TDF (0.93/100 PY), IRR (95% CI): 0.111 (0.024, 0.513), p=0.002451,3

qThe determination of efficacy was based on interim analyses when 50% of randomized participants completed 52 weeks of follow-up. Based on results, the study arms were unblinded, and this interim analysis became the primary analysis.

Safety and tolerability data1-3
Review the safety profile for YEZTUGO, including adverse reactions and injection site reactions (ISRs), and see how the frequency of ISRs decreased with subsequent doses.

The only twice-yearly dosing1,4
The first-and-only HIV prevention option that is 2 subcutaneous injections every 6 months, done in office within a ±2-week continuation dosing window from the scheduled injection date.
bHIV=background HIV; CI=confidence interval; DHHS=US Department of Health and Human Services; FTC/TAF=emtricitabine/tenofovir alafenamide fumarate; FTC/TDF=emtricitabine/tenofovir disoproxil fumarate; INSTI=integrase strand transfer inhibitor; IRR=incidence rate ratio; NRTI=nucleoside/nucleotide reverse transcriptase inhibitor; PY=person-years; SC=subcutaneous.
References:
- YEZTUGO. Prescribing information. Gilead Sciences, Inc.; 2025.
- Bekker LG, Das M, Abdool Karim Q, et al; PURPOSE 1 study team. Twice-yearly lenacapavir or daily F/TAF for HIV prevention in cisgender women. N Engl J Med. 2024;391(13):1179-1192.
- Kelley CF, Acevedo-Quiñones M, Agwu AL, et al; PURPOSE 2 study team. Twice-yearly lenacapavir for HIV prevention in men and gender-diverse persons. N Engl J Med. 2025;392(13):1261-1276.
- Full efficacy and safety results for Gilead investigational twice-yearly lenacapavir for HIV prevention presented at AIDS 2024. News release. Gilead Sciences, Inc.; July 24, 2024. Accessed January 6, 2025. https://www.gilead.com/news/news-details/2024/full-efficacy-and-safety-results-for-gilead-investigational-twice-yearly-lenacapavir-for-hiv-prevention-presented-at-aids-2024
- Gallardo-Cartagena J, Phanuphak N, Ndlovu N, et al. Global racial, ethnic, and gender diversity among participants enrolled in the PURPOSE 2 trial of lenacapavir for pre-exposure prophylaxis. Paper presented at: 5th HIV Research for Prevention Conference (HIVR4P); October 6-10, 2024; Lima, Peru.
- Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Clinicalinfo. Updated September 12, 2024. Accessed May 13, 2025. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf

