YEZTUGO is the first-and-only PrEP option that offers twice-yearly, in-office subcutaneous injections1-4
Confirm HIV-negative status prior to injecting YEZTUGO and additionally as clinically appropriate. In addition, counsel individuals about the importance of adherence to scheduled YEZTUGO dosing visits.1
Initiating YEZTUGO1


See additional information on delayed or missed doses
aTablets may be taken with or without food.
bUnless the injection schedule is interrupted.
How to administer YEZTUGO in your office1
6 key steps for every 6-month injection1
YEZTUGO is administered in office via 2 x 1.5-mL subcutaneous injections in the appropriate injection sites.
Please see the full Prescribing Information and Instructions for Use for more details on how to administer YEZTUGO.
Consider applying an ice pack prior to injections.5,g






Improper administration (intradermal injection) of lenacapavir has been associated with serious injection site reactions. Ensure YEZTUGO is only administered subcutaneously.
gThis consideration for injections is not specific to YEZTUGO. Follow the protocols for your institution.
hMake sure each vial contains yellow solution with no particles, contents are undamaged, and product is not expired. Remove vial cap and clean vial stopper with an alcohol wipe. Attach 18-G withdrawal needle and inject 1.5 mL of air into vial, withdraw all contents into the syringe. Remove 18-G withdrawal needle, attach 22-G injection needle, expel air bubbles, and prime to 1.5 mL.
Helpful injection training videos
Watch short videos on administering YEZTUGO, including details on how to inject it properly and how to counsel the individuals receiving it.
Key Steps to Inject
Learn how to administer YEZTUGO subcutaneously.
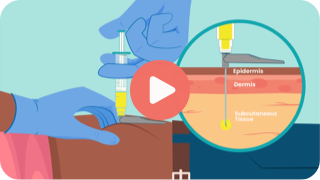
Key Steps to Inject
Learn how to administer YEZTUGO subcutaneously.
Key Steps to Inject. For US healthcare providers only.
Whether you’re injecting YEZTUGO for the first time or during a follow-up visit, this video walks you through an overview of the injection process.
This video is a companion to the full Prescribing Information and Instructions for Use for YEZTUGO, which you should review for complete details.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including Boxed Warning, available at YeztugoPI.com.
YEZTUGO is a twice-yearly injectable PrEP option that is given every six months (after initiation dosing).
When someone starts YEZTUGO, initiation dosing consists of two 1.5-milliliter subcutaneous injections (927 milligrams total), administered in office with two 300–milligram oral tablets. On Day 2, two more 300-milligram oral tablets are taken at home (with or without food).
Continuation dosing is administered in office with two subcutaneous injections every six months, or 26 weeks, from the date of the last injection, plus or minus two weeks.
Confirm negative HIV-1 status prior to each injection of YEZTUGO, and additionally as clinically appropriate.
See full Prescribing Information for recommended dosage modifications when YEZTUGO is used concomitantly with strong or moderate CYP3A inducers.
Before you open the kit, verify that the name on the kit matches that of the person receiving the injections, as well as confirm the date of the last YEZTUGO injection to make sure it is administered according to their dosing schedule.
Now let’s take a look at what’s inside the YEZTUGO injection kit.
Since each dose is delivered with two injections, each kit contains:
- Two single-dose vials (1.5 milliliters each)
- Two syringes
- Two withdrawal needles (18 gauge, 1.5 inch)
- Two injection needles (22 gauge, 0.5 inch)
- Prescribing Information
- Instructions for Use
Remember that each component is for single use only. The vials provided are ready to use, with no mixing or reconstitution necessary.
As you open the injection kit, check to make sure nothing is missing, damaged, or expired and that the vials contain a yellow solution with no particles. Do not use YEZTUGO injection if the solution is discolored or if it contains particulate matter.
If you need a replacement kit for any reason, contact Gilead Sciences at 1-800-GILEAD-5.
Now we’re ready to review how to administer YEZTUGO by injection.
Remember that initiation dosing consists of 2 subcutaneous injections and 2 oral tablets taken on Day 1, as well as 2 oral tablets on Day 2 (at home). If an individual misses the Day 2 oral loading dose (600 mg), have them take it as soon as possible. They should not take the Day 1 and Day 2 oral loading doses on the same day.
Consider applying an ice pack prior to injections. This consideration for injections is not specific to YEZTUGO. Follow the protocols for your institution.
For the subcutaneous injection of YEZTUGO, keep these 6 key steps in mind to help administer each 6-month injection.
Step 1: Ready the dose by preparing, assembling, and filling the syringe for injection.
Remove the vial cap and clean the stopper with an alcohol wipe.
Attach the pink 18-gauge, 1.5-inch withdrawal needle to the syringe.
Use the syringe to inject 1.5 milliliters of air into the vial before withdrawing all contents from the vial into the syringe.
Then replace the pink needle with the gray 22-gauge, 0.5-inch injection needle.
Expel any air bubbles from the syringe and prime it to 1.5 milliliters.
Once the solution has been drawn into the syringe, the injection should be administered as soon as possible.
Step 2: Pick an injection site. Before injecting someone with YEZTUGO, talk with them about choosing an appropriate site for each injection. Sites to choose from include the abdomen (at least 2 inches from the navel) or thigh. Keep in mind that the two injection sites should be at least 4 inches from each other.
When choosing a site together, find one that allows you to pinch enough skin to properly inject YEZTUGO subcutaneously. Think about the amount of body fat at each site, as areas with more body fat may give you more skin to pinch. Consider other factors for each individual as you help choose an injection site.
It’s important to pick a site that allows you to avoid injecting YEZTUGO into the dermis, as this could cause serious injection site reactions.
Once you agree on a site, clean it using the appropriate aseptic technique.
Step 3: Pinch the skin. Gently pinch a broad portion of skin at the injection site to help fully insert the needle into the subcutaneous layer where the drug depot forms. This pinch may help give you more subcutaneous tissue to target for the injection, especially in participants with thinner body habitus. Make sure the pinch is gentle to avoid injecting into compressed tissue.
Step 4: Find the right angle. Fully insert the needle at an angle between 45 and 90 degrees. A 90-degree angle is preferred.
This may help you inject directly into the subcutaneous layer. Avoid inserting the needle at less than a 45-degree angle.
Step 5: Take it slow and steady. YEZTUGO is a viscous solution that should be injected slowly and carefully. Remove the needle from the injection site at the same angle at which it was inserted.
Then gently apply sterile gauze to the injection site, as needed, for bleeding or residual solution that may leak onto the surface of the skin. Apply a bandage, as needed.
Once the first injection is done, repeat Steps 2 through 5 for the second injection. Please see full Prescribing Information for YEZTUGO and Instructions for Use for more details on how to prepare and administer YEZTUGO.
Remember that the second injection site must be at least 4 inches from the first site and 2 inches from the navel.
Once you finish using each injection kit component, remember to follow the appropriate steps to dispose of them, as well as any unused portion of the solution.
If you want a reminder on the key steps of the injection process, review this video along with the full Prescribing Information.
The injection kit for the next 6-month follow-up visit should be stored at a controlled room temperature. Keep the vials in the original carton until just prior to preparation of the injections in order to protect them from light.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.
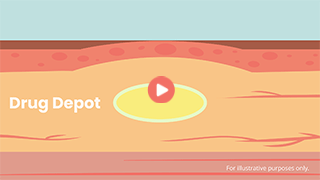
A Closer Look at the Drug Depot
See how a YEZTUGO repository forms at each injection site.
A Closer Look at the Drug Depot. For US healthcare providers only.
When you administer a subcutaneous injection of YEZTUGO, it forms what is called a drug depot under the skin.
This video helps you learn more about a drug depot and what someone in your care may feel at the injection site.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
As a twice-yearly PrEP option, YEZTUGO is administered every 6 months via subcutaneous injections after initiation dosing. You can learn more about initiation and continuation dosing for YEZTUGO at YEZTUGOdosing.com.
Let’s dive into one of those injection sites for a closer look at what happens beneath the skin whenever YEZTUGO is injected.
At each injection site, this viscous medication collects within the subcutaneous tissue to form what is known as a drug depot. Think of it as a localized storage area of YEZTUGO, where it’s slowly released over time. This gradual release contributes to the longer action of YEZTUGO.
At the injection site, a bump (or nodule) may be felt under the skin as a possible reaction to the drug depot.
With YEZTUGO, a bump may be felt at the injection site but may not be visible. However, with or without a bump, a drug depot forms at each injection site.
They may also be interested to know that, as YEZTUGO slowly releases over time, the drug depot shrinks.
Remind them that even if they developed a bump after this particular injection, experiences can vary from one injection to the next.
As a provider, you may want to know which injection site reactions (or ISRs) occurred, and how often these ISRs, including nodules, occurred in two phase 3 clinical trials of YEZTUGO: PURPOSE 1 and PURPOSE 2.
ISRs reported in greater than or equal to 2% of participants receiving YEZTUGO in either study were nodule, pain, induration, swelling, pruritus, erythema, bruising, and warmth. To learn more about these clinical trials, go to YEZTUGOhcp.com.
In each trial, discontinuations due to ISRs were low.
Nodules resolved more slowly than other ISRs. The median duration of nodules associated with the first injections of YEZTUGO was 350 days in PURPOSE 1 and 297 days in PURPOSE 2.
In terms of nodule size, the median of the maximum observed nodule diameter from each participant was 3 centimeters in PURPOSE 1 and PURPOSE 2.
Now that you know more about what you may expect at each site, let’s review some tips for learning and practicing proper injection technique at each site.
This includes keeping injection sites at least 4 inches from each other and 2 inches from the navel.
There are a couple of injection sites to choose from. Those sites can include the abdomen (again, at least 2 inches from the navel), or thigh.
When choosing a site, think about which areas have more fatty tissue to pinch when injecting YEZTUGO. It’s important to remember the proper injection technique, which includes gently pinching a broad portion of skin to target at the injection site.
You should fully insert the needle at a 90-degree angle, which is the preferred approach. This may help you inject fully into the subcutaneous layer. When someone has less fatty tissue to pinch, the angle of injection can be between 90 and 45 degrees, but do not inject at less than a 45-degree angle.
Remember that improper administration of YEZTUGO, such as intradermally, has been associated with serious injection site reactions. Ensure YEZTUGO is only administered subcutaneously.
You should also keep in mind that just as no 2 bodies are alike, no 2 injection sites are alike. The subcutaneous layer may be closer to the surface of the skin in some people—and in some sites—more than others.
You might also consider some strategies you typically use in clinical practice when administering a subcutaneous injection to help address injection site pain, such as using ice packs or analgesics as clinically appropriate.
These strategies are not specific to YEZTUGO. Follow the pain management protocol for your institution and as clinically appropriate. More tips and strategies will be discussed in another video in this series.
Now, let’s also discuss how you can talk with someone in your care about the drug depot and a possible bump or nodule.
Consider proactively talking about this at the injection visit while educating them about the use of YEZTUGO. Tell them that YEZTUGO collects under the skin at the injection site, where it is slowly released over time.
Some individuals may feel a bump as a possible reaction to the drug depot. It may also help to reassure them that, with or without a bump, YEZTUGO is stored under the skin at each injection site.
If they have any other questions or concerns that need to be addressed, including side effects, remind them to discuss them with you.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

Tips for Discussing Injections
Learn to discuss injections with those in your care..
Tips for Discussing Injections. For US healthcare providers only.
Once someone in your care is ready to receive YEZTUGO, there may be certain topics to discuss related to their subcutaneous injections.
This video offers tips on how to talk about the injection process with someone who has been prescribed YEZTUGO.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
Start by reminding them that YEZTUGO is a prescription medicine that is used to reduce the risk of HIV. This type of product is called PrEP, which stands for pre-exposure prophylaxis. YEZTUGO is a twice-yearly injectable PrEP option, which will be given to them in the office by a healthcare provider every 6 months, after initiation dosing.
Next, help them understand what to expect when starting on YEZTUGO. Tell them how the first 6-month doses will be different than the rest of their 6-month injection visits. Remind them that they will need to have a negative HIV-1 test prior to each injection of YEZTUGO, and additionally as clinically appropriate.
Explain that, on the first day, they will take two YEZTUGO pills (300 mg each) by mouth, along with two YEZTUGO injections, in your office. This will be followed by two more pills taken at home the next day. These pills may be taken with or without food. If an individual misses the Day 2 oral loading dose (600 mg), have them take it as soon as possible. They should not take the Day 1 and Day 2 oral loading doses on the same day.
After the starter doses, they will only get two injections every 6 months, and no more pills (when the schedule is followed as prescribed). See full Prescribing Information for recommended dosage modifications when YEZTUGO is administered with strong or moderate CYP3A inducers.
As a provider, you should review the steps in the injection process before you administer YEZTUGO. See the “Key Steps to Inject” episode of this video series as well as full Prescribing Information and Instructions for Use.
Remind them that it is important they attend their scheduled appointments to receive their subcutaneous injections of YEZTUGO every 6 months since missing YEZTUGO injections or pills increases their risk of getting HIV-1.
Let them know that each dose can be scheduled with you up to 2 weeks earlier or 2 weeks later than the scheduled injection date, 6 months from the last injection.
If they are not able to stay on schedule, let them know that there are options to help them avoid interrupting YEZTUGO. As a provider, you can learn more about these options in the full Prescribing Information.
Explain that ongoing testing is a part of HIV prevention. Remind them again that they will need to have a negative HIV-1 test prior to receiving YEZTUGO injections, and additionally as clinically appropriate.
It may be helpful for them to set reminders for their HIV-1 tests and their injection appointments.
As a provider, you may also consider voice and text reminders to individuals in your care.
Before they receive YEZTUGO, help them understand what they may notice after injections.
Explain to them that YEZTUGO injections are given under the skin. Some people may notice or feel a bump (or lump) at the injection site, while others may not. Having a bump is not required for YEZTUGO to work.
Talk with them about the possible side effects of YEZTUGO.
Let them know that the most common side effects of YEZTUGO are injection site reactions, headache, and nausea. Injection site reactions are experienced by many people who take YEZTUGO.
Injection site reactions may include a bump, pain, skin hardening, swelling, itching, redness, bruising, or warmth. If they develop a bump or hardened skin at the injection site, it may be felt but not seen and may take longer to go away than other injection site reactions. Let them know that these are not all of the possible side effects of YEZTUGO.
After their injections, remind them to tell you if they have any injection site reactions or other side effects.
Make sure you review the full Prescribing Information to understand potential side effects you may want to discuss with them.
Let’s review the Important Safety Information to learn more about YEZTUGO.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

Addressing Potential Injection Site Reactions
Learn about ISRs and tips for addressing them.
Addressing Potential Injection Site Reactions. For US healthcare providers only.
When administering subcutaneous injections of YEZTUGO, you may want to know more about potential injection site reactions (or ISRs).
This video offers more details about ISRs from two clinical trials, as well as strategies to help address one of these potential ISRs, specifically injection site pain.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including Boxed Warning, available at YeztugoPI.com.
Let’s start by talking about how often ISRs were reported in two phase 3 clinical trials: PURPOSE 1 and PURPOSE 2.
ISRs reported in greater than or equal to 2% of participants receiving YEZTUGO in either study were nodule, pain, induration, swelling, pruritus, erythema, bruising, and warmth. To learn more about these clinical trials, go to YEZTUGOhcp.com.
While ISRs were common and reported across both trials among people on YEZTUGO, the majority of ISRs were mild to moderate. No serious ISRs occurred in either PURPOSE 1 or PURPOSE 2.
In each trial, discontinuations due to ISRs were low.
So, now that you know what ISRs to watch for, let’s review some tips to keep in mind while injecting YEZTUGO.
Finding an appropriate injection site may take some careful consideration to help fully insert the needle into the subcutaneous layer where the drug depot forms.
Every person has a different body type. Pick a site that allows you to pinch enough skin to inject subcutaneously.
One of the most important steps you can take is to learn and practice the proper injection technique.
Improper administration of lenacapavir (in other words, by intradermal injection instead of subcutaneous injection) has been associated with serious ISRs. Remember, YEZTUGO should only be administered subcutaneously.
To review the steps in the injection process in more detail, see the “Key Steps to Inject” episode of this video series and see the full Prescribing Information and Instructions for Use for more details on how to prepare and administer YEZTUGO.
After someone receives their injections, remind them to report any ISRs they may experience at any time. If they have questions or concerns about ISRs or other adverse reactions, remind them to contact you.
One specific ISR you may be interested in helping to address is potential injection site pain. There may be some other techniques you’ve used in the past to address this pain during subcutaneous injections. Although these techniques are not specific to YEZTUGO, consider if they may be appropriate for some people receiving YEZTUGO injections.
For example, you may be used to rotating injection sites between visits. Injection sites include the abdomen (at least 2 inches from the navel), or thigh. Remember that the second injection site must be at least 4 inches from the first site.
Applying an ice pack to each site prior to injection may be another strategy you have used in the past. Consider keeping a few ice packs in your office and ready for your injection visits.
In addition to the techniques used in your experience with subcutaneous injections, there may be other methods for you to consider when addressing injection site pain.
One method could include counseling or coaching someone through the injection process to comfort or distract them from the potential pain during injection.
Pain mitigations for other injectables have also included using topical numbing creams before, or oral analgesics after the injection (if clinically appropriate).
If clinically indicated, you may also consider skin stimulation devices that are designed to decrease pain perception through local skin stimulation by applying both cold and vibration to the site. Remember that these strategies are not specific to YEZTUGO. Follow the pain management protocol for your institution.
After someone receives their YEZTUGO injections, remind them to tell you if they have any ISRs or other side effects.
You can also report any suspected adverse reactions to Gilead Sciences, Inc. at 1-800-GILEAD-5, or to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Let’s review some of what you learned today. Remember to carefully select an injection site that allows for proper injection. Before the next injection visit, refresh your memory and practice the proper injection technique. You may also consider other strategies (such as ice packs) that may help address injection site pain as clinically appropriate.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

Following the Dosing Schedule
See when to schedule injections and testing.
Following the Dosing Schedule. For US healthcare providers only.
Consistency is important for YEZTUGO, which is why it’s important for both you and someone in your care to understand the dosing schedule and how to follow it.
In this video, you’ll learn more about the YEZTUGO dosing schedule and get tips on helping someone track each of their scheduled visits.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including Boxed Warning, available at YeztugoPI.com.
Let’s start with a basic understanding of the typical schedule. As a twice-yearly injectable PrEP, YEZTUGO is administered every 6 months (or 26 weeks) by a healthcare provider in a clinical setting.
When someone starts YEZTUGO, the first 2 days are slightly different than the continuation dosing that follows.
That’s because on Day 1, during an in-office visit, two subcutaneous injections will be administered and a loading dose of two 300-mg oral tablets will be given.
On Day 2, at home, two more 300-mg oral tablets are taken. If an individual misses the Day 2 oral loading dose (600 mg), have them take it as soon as possible. They should not take the Day 1 and Day 2 oral loading doses on the same day.
After that, continuation dosing is administered every 6 months with 2 injections (with no oral tablets) when the dosing schedule is followed as prescribed. Ideally, continuation injections would be administered on the date that you schedule. However, a lot can change about someone’s plans in the 6 months between visits.
That’s why the dosing schedule is designed for flexibility in continuation dosing. When needed, YEZTUGO can be administered up to 2 weeks earlier or 2 weeks later than the scheduled injection date, 6 months from the last injection. It may be helpful to check in with each person before their next visit, to see if this flexibility would help them stay on schedule.
Remember that you must confirm a negative HIV-1 test prior to each injection of YEZTUGO, and additionally as clinically appropriate.
Now that you know more about the dosing schedule, how do you help someone follow that schedule for their 6-month injections?
It may help to give them some basic context about how YEZTUGO can only prevent HIV if it’s administered as prescribed.
It’s important to follow the dosing schedule as closely as possible to avoid interrupting their HIV prevention. To help them stay on schedule, consider staying in touch with them between visits.
Let’s look ahead a few months, just before their scheduled 6-month injection visit. If they do miss injections for whatever reason, there are steps you can take.
Make sure they notify you as soon as possible if their next appointment may need to change.
Let’s talk about that situation, where they anticipate a delay in their scheduled 6-month injection visit by more than 2 weeks. When that happens and it’s been 26 to 28 weeks since their last injection, YEZTUGO oral tablets may be used on an interim basis.
They would take one 300-mg oral tablet every 7 days for up to 6 months, until injections resume. They must resume the continuation injection dosing within 7 days after the last oral dose.
If someone accidentally misses a scheduled injection visit by more than 28 weeks and YEZTUGO tablets have not been taken, they should be clinically reassessed to ensure they’re appropriate to resume YEZTUGO and that they remain HIV-1 negative.
If they are HIV-1 negative and appropriate to resume, that person needs to restart the initiation dosing schedule with oral tablets and injections. After the initiation dosing, they will continue with continuation injection dosing. See the Prescribing Information for more details.
Remind them that they will need to be tested to confirm negative HIV-1 status prior to each YEZTUGO injection, and as clinically appropriate. What are some other ways that you can help someone follow their schedule?
You may want to recommend that they use several types of reminders to keep track of upcoming visits for HIV-1 testing and injections.
This includes writing dates on a calendar, using apps on their phone, or other methods they typically use to remember when to take other medications.
You may also consider sending them reminders, notifications, or calls to help remind them of their scheduled visits.
Tell them to contact you right away if they are unable to attend their scheduled appointments, and to discuss appropriate next steps.
Now that you have more details about the dosing schedule, let’s discuss a couple of situations where dosing modifications may be needed.
Supplemental doses of YEZTUGO are recommended for individuals initiating therapy with either strong or moderate CYP3A inducers.
Continue to administer once-every-6-months scheduled continuation dosing of YEZTUGO 927 mg subcutaneously (two 1.5-milliliter injections), plus administer supplemental doses of YEZTUGO as shown in the following tables. Strong CYP3A inducers may be initiated starting at least 2 days after YEZTUGO is first initiated, while moderate CYP3A inducers may be started any time after YEZTUGO is first initiated. See full Prescribing Information for more details.
Let’s review some of what you learned today. Remember to test to confirm negative HIV-1 status prior to injecting YEZTUGO and additionally thereafter as clinically appropriate. After the initiation dosing, YEZTUGO is administered by subcutaneous injection every 6 months. Continuation dosing is flexible and options are available if the scheduled injection is missed (or anticipated to be delayed). Also, reminders are an important way to help someone remember their scheduled visits. As needed, follow instructions for dosing modifications per the full Prescribing Information.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.
Key Steps to Inject. For US healthcare providers only.
Whether you’re injecting YEZTUGO for the first time or during a follow-up visit, this video walks you through an overview of the injection process.
This video is a companion to the full Prescribing Information and Instructions for Use for YEZTUGO, which you should review for complete details.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including Boxed Warning, available at YeztugoPI.com.
YEZTUGO is a twice-yearly injectable PrEP option that is given every six months (after initiation dosing).
When someone starts YEZTUGO, initiation dosing consists of two 1.5-milliliter subcutaneous injections (927 milligrams total), administered in office with two 300–milligram oral tablets. On Day 2, two more 300-milligram oral tablets are taken at home (with or without food).
Continuation dosing is administered in office with two subcutaneous injections every six months, or 26 weeks, from the date of the last injection, plus or minus two weeks.
Confirm negative HIV-1 status prior to each injection of YEZTUGO, and additionally as clinically appropriate.
See full Prescribing Information for recommended dosage modifications when YEZTUGO is used concomitantly with strong or moderate CYP3A inducers.
Before you open the kit, verify that the name on the kit matches that of the person receiving the injections, as well as confirm the date of the last YEZTUGO injection to make sure it is administered according to their dosing schedule.
Now let’s take a look at what’s inside the YEZTUGO injection kit.
Since each dose is delivered with two injections, each kit contains:
- Two single-dose vials (1.5 milliliters each)
- Two syringes
- Two withdrawal needles (18 gauge, 1.5 inch)
- Two injection needles (22 gauge, 0.5 inch)
- Prescribing Information
- Instructions for Use
Remember that each component is for single use only. The vials provided are ready to use, with no mixing or reconstitution necessary.
As you open the injection kit, check to make sure nothing is missing, damaged, or expired and that the vials contain a yellow solution with no particles. Do not use YEZTUGO injection if the solution is discolored or if it contains particulate matter.
If you need a replacement kit for any reason, contact Gilead Sciences at 1-800-GILEAD-5.
Now we’re ready to review how to administer YEZTUGO by injection.
Remember that initiation dosing consists of 2 subcutaneous injections and 2 oral tablets taken on Day 1, as well as 2 oral tablets on Day 2 (at home). If an individual misses the Day 2 oral loading dose (600 mg), have them take it as soon as possible. They should not take the Day 1 and Day 2 oral loading doses on the same day.
Consider applying an ice pack prior to injections. This consideration for injections is not specific to YEZTUGO. Follow the protocols for your institution.
For the subcutaneous injection of YEZTUGO, keep these 6 key steps in mind to help administer each 6-month injection.
Step 1: Ready the dose by preparing, assembling, and filling the syringe for injection.
Remove the vial cap and clean the stopper with an alcohol wipe.
Attach the pink 18-gauge, 1.5-inch withdrawal needle to the syringe.
Use the syringe to inject 1.5 milliliters of air into the vial before withdrawing all contents from the vial into the syringe.
Then replace the pink needle with the gray 22-gauge, 0.5-inch injection needle.
Expel any air bubbles from the syringe and prime it to 1.5 milliliters.
Once the solution has been drawn into the syringe, the injection should be administered as soon as possible.
Step 2: Pick an injection site. Before injecting someone with YEZTUGO, talk with them about choosing an appropriate site for each injection. Sites to choose from include the abdomen (at least 2 inches from the navel) or thigh. Keep in mind that the two injection sites should be at least 4 inches from each other.
When choosing a site together, find one that allows you to pinch enough skin to properly inject YEZTUGO subcutaneously. Think about the amount of body fat at each site, as areas with more body fat may give you more skin to pinch. Consider other factors for each individual as you help choose an injection site.
It’s important to pick a site that allows you to avoid injecting YEZTUGO into the dermis, as this could cause serious injection site reactions.
Once you agree on a site, clean it using the appropriate aseptic technique.
Step 3: Pinch the skin. Gently pinch a broad portion of skin at the injection site to help fully insert the needle into the subcutaneous layer where the drug depot forms. This pinch may help give you more subcutaneous tissue to target for the injection, especially in participants with thinner body habitus. Make sure the pinch is gentle to avoid injecting into compressed tissue.
Step 4: Find the right angle. Fully insert the needle at an angle between 45 and 90 degrees. A 90-degree angle is preferred.
This may help you inject directly into the subcutaneous layer. Avoid inserting the needle at less than a 45-degree angle.
Step 5: Take it slow and steady. YEZTUGO is a viscous solution that should be injected slowly and carefully. Remove the needle from the injection site at the same angle at which it was inserted.
Then gently apply sterile gauze to the injection site, as needed, for bleeding or residual solution that may leak onto the surface of the skin. Apply a bandage, as needed.
Once the first injection is done, repeat Steps 2 through 5 for the second injection. Please see full Prescribing Information for YEZTUGO and Instructions for Use for more details on how to prepare and administer YEZTUGO.
Remember that the second injection site must be at least 4 inches from the first site and 2 inches from the navel.
Once you finish using each injection kit component, remember to follow the appropriate steps to dispose of them, as well as any unused portion of the solution.
If you want a reminder on the key steps of the injection process, review this video along with the full Prescribing Information.
The injection kit for the next 6-month follow-up visit should be stored at a controlled room temperature. Keep the vials in the original carton until just prior to preparation of the injections in order to protect them from light.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

Key Steps to Inject
Learn how to administer YEZTUGO subcutaneously.
Key Steps to Inject. For US healthcare providers only.
Whether you’re injecting YEZTUGO for the first time or during a follow-up visit, this video walks you through an overview of the injection process.
This video is a companion to the full Prescribing Information and Instructions for Use for YEZTUGO, which you should review for complete details.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including Boxed Warning, available at YeztugoPI.com.
YEZTUGO is a twice-yearly injectable PrEP option that is given every six months (after initiation dosing).
When someone starts YEZTUGO, initiation dosing consists of two 1.5-milliliter subcutaneous injections (927 milligrams total), administered in office with two 300–milligram oral tablets. On Day 2, two more 300-milligram oral tablets are taken at home (with or without food).
Continuation dosing is administered in office with two subcutaneous injections every six months, or 26 weeks, from the date of the last injection, plus or minus two weeks.
Confirm negative HIV-1 status prior to each injection of YEZTUGO, and additionally as clinically appropriate.
See full Prescribing Information for recommended dosage modifications when YEZTUGO is used concomitantly with strong or moderate CYP3A inducers.
Before you open the kit, verify that the name on the kit matches that of the person receiving the injections, as well as confirm the date of the last YEZTUGO injection to make sure it is administered according to their dosing schedule.
Now let’s take a look at what’s inside the YEZTUGO injection kit.
Since each dose is delivered with two injections, each kit contains:
- Two single-dose vials (1.5 milliliters each)
- Two syringes
- Two withdrawal needles (18 gauge, 1.5 inch)
- Two injection needles (22 gauge, 0.5 inch)
- Prescribing Information
- Instructions for Use
Remember that each component is for single use only. The vials provided are ready to use, with no mixing or reconstitution necessary.
As you open the injection kit, check to make sure nothing is missing, damaged, or expired and that the vials contain a yellow solution with no particles. Do not use YEZTUGO injection if the solution is discolored or if it contains particulate matter.
If you need a replacement kit for any reason, contact Gilead Sciences at 1-800-GILEAD-5.
Now we’re ready to review how to administer YEZTUGO by injection.
Remember that initiation dosing consists of 2 subcutaneous injections and 2 oral tablets taken on Day 1, as well as 2 oral tablets on Day 2 (at home). If an individual misses the Day 2 oral loading dose (600 mg), have them take it as soon as possible. They should not take the Day 1 and Day 2 oral loading doses on the same day.
Consider applying an ice pack prior to injections. This consideration for injections is not specific to YEZTUGO. Follow the protocols for your institution.
For the subcutaneous injection of YEZTUGO, keep these 6 key steps in mind to help administer each 6-month injection.
Step 1: Ready the dose by preparing, assembling, and filling the syringe for injection.
Remove the vial cap and clean the stopper with an alcohol wipe.
Attach the pink 18-gauge, 1.5-inch withdrawal needle to the syringe.
Use the syringe to inject 1.5 milliliters of air into the vial before withdrawing all contents from the vial into the syringe.
Then replace the pink needle with the gray 22-gauge, 0.5-inch injection needle.
Expel any air bubbles from the syringe and prime it to 1.5 milliliters.
Once the solution has been drawn into the syringe, the injection should be administered as soon as possible.
Step 2: Pick an injection site. Before injecting someone with YEZTUGO, talk with them about choosing an appropriate site for each injection. Sites to choose from include the abdomen (at least 2 inches from the navel) or thigh. Keep in mind that the two injection sites should be at least 4 inches from each other.
When choosing a site together, find one that allows you to pinch enough skin to properly inject YEZTUGO subcutaneously. Think about the amount of body fat at each site, as areas with more body fat may give you more skin to pinch. Consider other factors for each individual as you help choose an injection site.
It’s important to pick a site that allows you to avoid injecting YEZTUGO into the dermis, as this could cause serious injection site reactions.
Once you agree on a site, clean it using the appropriate aseptic technique.
Step 3: Pinch the skin. Gently pinch a broad portion of skin at the injection site to help fully insert the needle into the subcutaneous layer where the drug depot forms. This pinch may help give you more subcutaneous tissue to target for the injection, especially in participants with thinner body habitus. Make sure the pinch is gentle to avoid injecting into compressed tissue.
Step 4: Find the right angle. Fully insert the needle at an angle between 45 and 90 degrees. A 90-degree angle is preferred.
This may help you inject directly into the subcutaneous layer. Avoid inserting the needle at less than a 45-degree angle.
Step 5: Take it slow and steady. YEZTUGO is a viscous solution that should be injected slowly and carefully. Remove the needle from the injection site at the same angle at which it was inserted.
Then gently apply sterile gauze to the injection site, as needed, for bleeding or residual solution that may leak onto the surface of the skin. Apply a bandage, as needed.
Once the first injection is done, repeat Steps 2 through 5 for the second injection. Please see full Prescribing Information for YEZTUGO and Instructions for Use for more details on how to prepare and administer YEZTUGO.
Remember that the second injection site must be at least 4 inches from the first site and 2 inches from the navel.
Once you finish using each injection kit component, remember to follow the appropriate steps to dispose of them, as well as any unused portion of the solution.
If you want a reminder on the key steps of the injection process, review this video along with the full Prescribing Information.
The injection kit for the next 6-month follow-up visit should be stored at a controlled room temperature. Keep the vials in the original carton until just prior to preparation of the injections in order to protect them from light.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

A Closer Look at the Drug Depot
See how a YEZTUGO repository forms at each injection site.
A Closer Look at the Drug Depot. For US healthcare providers only.
When you administer a subcutaneous injection of YEZTUGO, it forms what is called a drug depot under the skin.
This video helps you learn more about a drug depot and what someone in your care may feel at the injection site.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
As a twice-yearly PrEP option, YEZTUGO is administered every 6 months via subcutaneous injections after initiation dosing. You can learn more about initiation and continuation dosing for YEZTUGO at YEZTUGOdosing.com.
Let’s dive into one of those injection sites for a closer look at what happens beneath the skin whenever YEZTUGO is injected.
At each injection site, this viscous medication collects within the subcutaneous tissue to form what is known as a drug depot. Think of it as a localized storage area of YEZTUGO, where it’s slowly released over time. This gradual release contributes to the longer action of YEZTUGO.
At the injection site, a bump (or nodule) may be felt under the skin as a possible reaction to the drug depot.
With YEZTUGO, a bump may be felt at the injection site but may not be visible. However, with or without a bump, a drug depot forms at each injection site.
They may also be interested to know that, as YEZTUGO slowly releases over time, the drug depot shrinks.
Remind them that even if they developed a bump after this particular injection, experiences can vary from one injection to the next.
As a provider, you may want to know which injection site reactions (or ISRs) occurred, and how often these ISRs, including nodules, occurred in two phase 3 clinical trials of YEZTUGO: PURPOSE 1 and PURPOSE 2.
ISRs reported in greater than or equal to 2% of participants receiving YEZTUGO in either study were nodule, pain, induration, swelling, pruritus, erythema, bruising, and warmth. To learn more about these clinical trials, go to YEZTUGOhcp.com.
In each trial, discontinuations due to ISRs were low.
Nodules resolved more slowly than other ISRs. The median duration of nodules associated with the first injections of YEZTUGO was 350 days in PURPOSE 1 and 297 days in PURPOSE 2.
In terms of nodule size, the median of the maximum observed nodule diameter from each participant was 3 centimeters in PURPOSE 1 and PURPOSE 2.
Now that you know more about what you may expect at each site, let’s review some tips for learning and practicing proper injection technique at each site.
This includes keeping injection sites at least 4 inches from each other and 2 inches from the navel.
There are a couple of injection sites to choose from. Those sites can include the abdomen (again, at least 2 inches from the navel), or thigh.
When choosing a site, think about which areas have more fatty tissue to pinch when injecting YEZTUGO. It’s important to remember the proper injection technique, which includes gently pinching a broad portion of skin to target at the injection site.
You should fully insert the needle at a 90-degree angle, which is the preferred approach. This may help you inject fully into the subcutaneous layer. When someone has less fatty tissue to pinch, the angle of injection can be between 90 and 45 degrees, but do not inject at less than a 45-degree angle.
Remember that improper administration of YEZTUGO, such as intradermally, has been associated with serious injection site reactions. Ensure YEZTUGO is only administered subcutaneously.
You should also keep in mind that just as no 2 bodies are alike, no 2 injection sites are alike. The subcutaneous layer may be closer to the surface of the skin in some people—and in some sites—more than others.
You might also consider some strategies you typically use in clinical practice when administering a subcutaneous injection to help address injection site pain, such as using ice packs or analgesics as clinically appropriate.
These strategies are not specific to YEZTUGO. Follow the pain management protocol for your institution and as clinically appropriate. More tips and strategies will be discussed in another video in this series.
Now, let’s also discuss how you can talk with someone in your care about the drug depot and a possible bump or nodule.
Consider proactively talking about this at the injection visit while educating them about the use of YEZTUGO. Tell them that YEZTUGO collects under the skin at the injection site, where it is slowly released over time.
Some individuals may feel a bump as a possible reaction to the drug depot. It may also help to reassure them that, with or without a bump, YEZTUGO is stored under the skin at each injection site.
If they have any other questions or concerns that need to be addressed, including side effects, remind them to discuss them with you.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

Tips for Discussing Injections
Learn to discuss injections with those in your care..
Tips for Discussing Injections. For US healthcare providers only.
Once someone in your care is ready to receive YEZTUGO, there may be certain topics to discuss related to their subcutaneous injections.
This video offers tips on how to talk about the injection process with someone who has been prescribed YEZTUGO.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
Start by reminding them that YEZTUGO is a prescription medicine that is used to reduce the risk of HIV. This type of product is called PrEP, which stands for pre-exposure prophylaxis. YEZTUGO is a twice-yearly injectable PrEP option, which will be given to them in the office by a healthcare provider every 6 months, after initiation dosing.
Next, help them understand what to expect when starting on YEZTUGO. Tell them how the first 6-month doses will be different than the rest of their 6-month injection visits. Remind them that they will need to have a negative HIV-1 test prior to each injection of YEZTUGO, and additionally as clinically appropriate.
Explain that, on the first day, they will take two YEZTUGO pills (300 mg each) by mouth, along with two YEZTUGO injections, in your office. This will be followed by two more pills taken at home the next day. These pills may be taken with or without food. If an individual misses the Day 2 oral loading dose (600 mg), have them take it as soon as possible. They should not take the Day 1 and Day 2 oral loading doses on the same day.
After the starter doses, they will only get two injections every 6 months, and no more pills (when the schedule is followed as prescribed). See full Prescribing Information for recommended dosage modifications when YEZTUGO is administered with strong or moderate CYP3A inducers.
As a provider, you should review the steps in the injection process before you administer YEZTUGO. See the “Key Steps to Inject” episode of this video series as well as full Prescribing Information and Instructions for Use.
Remind them that it is important they attend their scheduled appointments to receive their subcutaneous injections of YEZTUGO every 6 months since missing YEZTUGO injections or pills increases their risk of getting HIV-1.
Let them know that each dose can be scheduled with you up to 2 weeks earlier or 2 weeks later than the scheduled injection date, 6 months from the last injection.
If they are not able to stay on schedule, let them know that there are options to help them avoid interrupting YEZTUGO. As a provider, you can learn more about these options in the full Prescribing Information.
Explain that ongoing testing is a part of HIV prevention. Remind them again that they will need to have a negative HIV-1 test prior to receiving YEZTUGO injections, and additionally as clinically appropriate.
It may be helpful for them to set reminders for their HIV-1 tests and their injection appointments.
As a provider, you may also consider voice and text reminders to individuals in your care.
Before they receive YEZTUGO, help them understand what they may notice after injections.
Explain to them that YEZTUGO injections are given under the skin. Some people may notice or feel a bump (or lump) at the injection site, while others may not. Having a bump is not required for YEZTUGO to work.
Talk with them about the possible side effects of YEZTUGO.
Let them know that the most common side effects of YEZTUGO are injection site reactions, headache, and nausea. Injection site reactions are experienced by many people who take YEZTUGO.
Injection site reactions may include a bump, pain, skin hardening, swelling, itching, redness, bruising, or warmth. If they develop a bump or hardened skin at the injection site, it may be felt but not seen and may take longer to go away than other injection site reactions. Let them know that these are not all of the possible side effects of YEZTUGO.
After their injections, remind them to tell you if they have any injection site reactions or other side effects.
Make sure you review the full Prescribing Information to understand potential side effects you may want to discuss with them.
Let’s review the Important Safety Information to learn more about YEZTUGO.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

Addressing Potential Injection Site Reactions
Learn about ISRs and tips for addressing them.
Addressing Potential Injection Site Reactions. For US healthcare providers only.
When administering subcutaneous injections of YEZTUGO, you may want to know more about potential injection site reactions (or ISRs).
This video offers more details about ISRs from two clinical trials, as well as strategies to help address one of these potential ISRs, specifically injection site pain.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including Boxed Warning, available at YeztugoPI.com.
Let’s start by talking about how often ISRs were reported in two phase 3 clinical trials: PURPOSE 1 and PURPOSE 2.
ISRs reported in greater than or equal to 2% of participants receiving YEZTUGO in either study were nodule, pain, induration, swelling, pruritus, erythema, bruising, and warmth. To learn more about these clinical trials, go to YEZTUGOhcp.com.
While ISRs were common and reported across both trials among people on YEZTUGO, the majority of ISRs were mild to moderate. No serious ISRs occurred in either PURPOSE 1 or PURPOSE 2.
In each trial, discontinuations due to ISRs were low.
So, now that you know what ISRs to watch for, let’s review some tips to keep in mind while injecting YEZTUGO.
Finding an appropriate injection site may take some careful consideration to help fully insert the needle into the subcutaneous layer where the drug depot forms.
Every person has a different body type. Pick a site that allows you to pinch enough skin to inject subcutaneously.
One of the most important steps you can take is to learn and practice the proper injection technique.
Improper administration of lenacapavir (in other words, by intradermal injection instead of subcutaneous injection) has been associated with serious ISRs. Remember, YEZTUGO should only be administered subcutaneously.
To review the steps in the injection process in more detail, see the “Key Steps to Inject” episode of this video series and see the full Prescribing Information and Instructions for Use for more details on how to prepare and administer YEZTUGO.
After someone receives their injections, remind them to report any ISRs they may experience at any time. If they have questions or concerns about ISRs or other adverse reactions, remind them to contact you.
One specific ISR you may be interested in helping to address is potential injection site pain. There may be some other techniques you’ve used in the past to address this pain during subcutaneous injections. Although these techniques are not specific to YEZTUGO, consider if they may be appropriate for some people receiving YEZTUGO injections.
For example, you may be used to rotating injection sites between visits. Injection sites include the abdomen (at least 2 inches from the navel), or thigh. Remember that the second injection site must be at least 4 inches from the first site.
Applying an ice pack to each site prior to injection may be another strategy you have used in the past. Consider keeping a few ice packs in your office and ready for your injection visits.
In addition to the techniques used in your experience with subcutaneous injections, there may be other methods for you to consider when addressing injection site pain.
One method could include counseling or coaching someone through the injection process to comfort or distract them from the potential pain during injection.
Pain mitigations for other injectables have also included using topical numbing creams before, or oral analgesics after the injection (if clinically appropriate).
If clinically indicated, you may also consider skin stimulation devices that are designed to decrease pain perception through local skin stimulation by applying both cold and vibration to the site. Remember that these strategies are not specific to YEZTUGO. Follow the pain management protocol for your institution.
After someone receives their YEZTUGO injections, remind them to tell you if they have any ISRs or other side effects.
You can also report any suspected adverse reactions to Gilead Sciences, Inc. at 1-800-GILEAD-5, or to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Let’s review some of what you learned today. Remember to carefully select an injection site that allows for proper injection. Before the next injection visit, refresh your memory and practice the proper injection technique. You may also consider other strategies (such as ice packs) that may help address injection site pain as clinically appropriate.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.

Following the Dosing Schedule
See when to schedule injections and testing.
Following the Dosing Schedule. For US healthcare providers only.
Consistency is important for YEZTUGO, which is why it’s important for both you and someone in your care to understand the dosing schedule and how to follow it.
In this video, you’ll learn more about the YEZTUGO dosing schedule and get tips on helping someone track each of their scheduled visits.
Before we begin, let’s review the indication and some Important Safety Information about YEZTUGO.
Indication
YEZTUGO is indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) kilograms who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Please see additional Important Safety Information later in this video and accompanying full Prescribing Information for YEZTUGO, including Boxed Warning, available at YeztugoPI.com.
Let’s start with a basic understanding of the typical schedule. As a twice-yearly injectable PrEP, YEZTUGO is administered every 6 months (or 26 weeks) by a healthcare provider in a clinical setting.
When someone starts YEZTUGO, the first 2 days are slightly different than the continuation dosing that follows.
That’s because on Day 1, during an in-office visit, two subcutaneous injections will be administered and a loading dose of two 300-mg oral tablets will be given.
On Day 2, at home, two more 300-mg oral tablets are taken. If an individual misses the Day 2 oral loading dose (600 mg), have them take it as soon as possible. They should not take the Day 1 and Day 2 oral loading doses on the same day.
After that, continuation dosing is administered every 6 months with 2 injections (with no oral tablets) when the dosing schedule is followed as prescribed. Ideally, continuation injections would be administered on the date that you schedule. However, a lot can change about someone’s plans in the 6 months between visits.
That’s why the dosing schedule is designed for flexibility in continuation dosing. When needed, YEZTUGO can be administered up to 2 weeks earlier or 2 weeks later than the scheduled injection date, 6 months from the last injection. It may be helpful to check in with each person before their next visit, to see if this flexibility would help them stay on schedule.
Remember that you must confirm a negative HIV-1 test prior to each injection of YEZTUGO, and additionally as clinically appropriate.
Now that you know more about the dosing schedule, how do you help someone follow that schedule for their 6-month injections?
It may help to give them some basic context about how YEZTUGO can only prevent HIV if it’s administered as prescribed.
It’s important to follow the dosing schedule as closely as possible to avoid interrupting their HIV prevention. To help them stay on schedule, consider staying in touch with them between visits.
Let’s look ahead a few months, just before their scheduled 6-month injection visit. If they do miss injections for whatever reason, there are steps you can take.
Make sure they notify you as soon as possible if their next appointment may need to change.
Let’s talk about that situation, where they anticipate a delay in their scheduled 6-month injection visit by more than 2 weeks. When that happens and it’s been 26 to 28 weeks since their last injection, YEZTUGO oral tablets may be used on an interim basis.
They would take one 300-mg oral tablet every 7 days for up to 6 months, until injections resume. They must resume the continuation injection dosing within 7 days after the last oral dose.
If someone accidentally misses a scheduled injection visit by more than 28 weeks and YEZTUGO tablets have not been taken, they should be clinically reassessed to ensure they’re appropriate to resume YEZTUGO and that they remain HIV-1 negative.
If they are HIV-1 negative and appropriate to resume, that person needs to restart the initiation dosing schedule with oral tablets and injections. After the initiation dosing, they will continue with continuation injection dosing. See the Prescribing Information for more details.
Remind them that they will need to be tested to confirm negative HIV-1 status prior to each YEZTUGO injection, and as clinically appropriate. What are some other ways that you can help someone follow their schedule?
You may want to recommend that they use several types of reminders to keep track of upcoming visits for HIV-1 testing and injections.
This includes writing dates on a calendar, using apps on their phone, or other methods they typically use to remember when to take other medications.
You may also consider sending them reminders, notifications, or calls to help remind them of their scheduled visits.
Tell them to contact you right away if they are unable to attend their scheduled appointments, and to discuss appropriate next steps.
Now that you have more details about the dosing schedule, let’s discuss a couple of situations where dosing modifications may be needed.
Supplemental doses of YEZTUGO are recommended for individuals initiating therapy with either strong or moderate CYP3A inducers.
Continue to administer once-every-6-months scheduled continuation dosing of YEZTUGO 927 mg subcutaneously (two 1.5-milliliter injections), plus administer supplemental doses of YEZTUGO as shown in the following tables. Strong CYP3A inducers may be initiated starting at least 2 days after YEZTUGO is first initiated, while moderate CYP3A inducers may be started any time after YEZTUGO is first initiated. See full Prescribing Information for more details.
Let’s review some of what you learned today. Remember to test to confirm negative HIV-1 status prior to injecting YEZTUGO and additionally thereafter as clinically appropriate. After the initiation dosing, YEZTUGO is administered by subcutaneous injection every 6 months. Continuation dosing is flexible and options are available if the scheduled injection is missed (or anticipated to be delayed). Also, reminders are an important way to help someone remember their scheduled visits. As needed, follow instructions for dosing modifications per the full Prescribing Information.
Important Safety Information (continued)
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
-
Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING, available at YeztugoPI.com.
For more information about YEZTUGO, visit YEZTUGOhcp.com.
Strategies to help address injection site pain
Consider the following for injection site pain1,5-7:



Pain occurred in 31% and 56% of participants on YEZTUGO in the PURPOSE 1 and PURPOSE 2 trials, respectively. Please click to see additional information on injection site reactions.
iThese strategies are not specific to YEZTUGO. Follow the pain management protocol for your institution.
What to expect from a YEZTUGO injection1:
When administering YEZTUGO, it is important to discuss 2 key aspects of the drug depot that forms following injection:
- The drug depot collects under the skin at the injection site
- Sometimes the drug depot may be felt as a bump/nodule,j but may not be visible
jNodules occurred in 64% and 63% of participants on YEZTUGO in the PURPOSE 1 and PURPOSE 2 trials, respectively.


Multiple ways to acquire YEZTUGO
There are several ways your office may acquire
YEZTUGO for the individuals in your care.

Important resources for your office
Access materials and support to help initiate individuals in your practice on YEZTUGO, along with helpful resources for your staff and practice.
Important Safety Information
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF YEZTUGO IN UNDIAGNOSED HIV-1 INFECTION
- Individuals must be tested for HIV-1 infection prior to initiating YEZTUGO, and with each subsequent injection of YEZTUGO, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with use of YEZTUGO by individuals with undiagnosed HIV-1 infection. Do not initiate YEZTUGO unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving YEZTUGO must transition to a complete HIV-1 treatment regimen.
Contraindications
- YEZTUGO is contraindicated in individuals with unknown or positive HIV-1 status.
Warnings and precautions
- Comprehensive risk management:
- Use YEZTUGO to reduce the risk of HIV-1 acquisition as part of a comprehensive prevention strategy including adherence to the administration schedule and safer sex practices, including condoms, to reduce the risk of sexually transmitted infections (STIs).
- HIV-1 acquisition risk includes behavioral, biological, or epidemiologic factors including, but not limited to, condomless sex, past or present STIs, self-identified HIV risk, having sexual partners of unknown HIV-1 viremic status, or sexual activity in a high-prevalence area or network. Counsel individuals on the use of other prevention methods to help reduce their risk.
- Use YEZTUGO only in individuals confirmed to be HIV-1 negative. Evaluate for current or recent signs or symptoms consistent with HIV-1 infection. Confirm HIV-1 negative status prior to initiating, prior to each subsequent injection, and as clinically appropriate.
- Potential risk of resistance:
- There is a potential risk of developing resistance to YEZTUGO if an individual acquires HIV-1 before or when receiving YEZTUGO, or following discontinuation. HIV-1 resistance substitutions may emerge in individuals with undiagnosed HIV-1 infection taking only YEZTUGO, because YEZTUGO alone is not a complete regimen for HIV-1 treatment.
- To minimize this risk, it is essential to test before each injection and additionally as clinically appropriate. Individuals confirmed to have HIV-1 must immediately begin a complete HIV-1 treatment regimen.
- Alternative forms of PrEP should be considered after discontinuation of YEZTUGO for those who are at continuing risk of HIV-1 acquisition and should be initiated within 28 weeks of the last YEZTUGO injection.
- Long-acting properties and potential associated risks:
- Residual concentrations of YEZTUGO may remain in systemic circulation for up to 12 months or longer after the last injection.
- Select individuals who agree to the required injection dosing schedule because nonadherence or missed doses could lead to HIV-1 acquisition and development of resistance.
- Serious injection site reactions: Improper administration (intradermal injection) has been associated with serious injection site reactions, including necrosis and ulcer. Only administer YEZTUGO subcutaneously.
Adverse reactions
- Most common adverse reactions (≥5%) in YEZTUGO clinical trials were injection site reactions, headache, and nausea.
Drug interactions
- Strong or moderate CYP3A inducers may significantly decrease YEZTUGO concentrations. Dosage modifications are recommended when initiating these inducers.
- It is not recommended to use YEZTUGO with combined P-gp, UGT1A1, and strong CYP3A inhibitors.
- Coadministration of YEZTUGO with sensitive substrates of CYP3A or P-gp may increase their concentrations and result in the increased risk of their adverse events. YEZTUGO may increase the exposure of drugs primarily metabolized by CYP3A initiated within 9 months after the last injection of YEZTUGO.
Dosage and administration
- HIV screening: Test for HIV-1 infection prior to initiating, prior to each subsequent injection, and as clinically appropriate using an approved or cleared test for the diagnosis of acute or primary HIV-1 infection.
- Dosage: Initiation dosing (injections and tablets) followed by once-every-6-months continuation injection dosing. Tablets may be taken with or without food.
- Initiation: Day 1: 927 mg by subcutaneous injection (2 x 1.5-mL injections) and 600 mg orally (2 x 300-mg tablets). Day 2: 600 mg orally.
- Continuation: 927 mg by subcutaneous injection every 6 months (26 weeks) from date of last injection ±2 weeks.
- Anticipated delayed injections: If scheduled 6-month injection is anticipated to be delayed by more than 2 weeks, YEZTUGO tablets may be taken on an interim basis (for up to 6 months) until injections resume. Dosage is 300 mg orally (1 x 300-mg tablet) once every 7 days. Resume continuation injections within 7 days of the last oral dose.
- Missed injections: If more than 28 weeks have elapsed since the last injection and YEZTUGO tablets have not been taken, restart with initiation dosing if clinically appropriate.
- Dosage modifications of YEZTUGO are recommended when initiating with strong or moderate CYP3A inducers. Consult the full Prescribing Information for recommendations.
Indication
YEZTUGO is indicated for pre‑exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents (≥35 kg) who are at risk for HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating YEZTUGO.
Please see full Prescribing Information for YEZTUGO, including BOXED WARNING.
References:
- YEZTUGO. Prescribing information. Gilead Sciences, Inc.; 2025.
- Bekker LG, Das M, Abdool Karim Q, et al; PURPOSE 1 study team. Twice-yearly lenacapavir or daily F/TAF for HIV prevention in cisgender women. N Engl J Med. 2024;391(13):1179-1192.
- Kelley CF, Acevedo-Quiñones M, Agwu AL, et al; PURPOSE 2 study team. Twice-yearly lenacapavir for HIV prevention in men and gender-diverse persons. N Engl J Med. 2025;392(13):1261-1276.
- Full efficacy and safety results for Gilead investigational twice-yearly lenacapavir for HIV prevention presented at AIDS 2024. News release. Gilead Sciences, Inc.; July 24, 2024. Accessed January 6, 2025. https://www.gilead.com/news/news-details/2024/full-efficacy-and-safety-results-for-gilead-investigational-twice-yearly-lenacapavir-for-hiv-prevention-presented-at-aids-2024
- Wang H, Guan J, Zhang X, et al. Effect of cold application on the pain and bruising in patients with subcutaneous injection of low-molecular-weight heparin: a meta-analysis. Clin Appl Thromb Hemost. 2020;26:1076029620905349.
- Rava J, Rosenau KA, Wilkie K, Bernacki J, Curcio E, Kuo A. The needle anxiety program: a patient-centered initiative for individuals with developmental disabilities. Cureus. 2023;16(7):e42253.
- Sivri Bilgen B, Balci S. The effect on pain of Buzzy® and ShotBlocker® during the administration of intramuscular injections to children: a randomized controlled trial. J Korean Acad Nurs. 2019;49(4):486-494.

